 |
 |
|
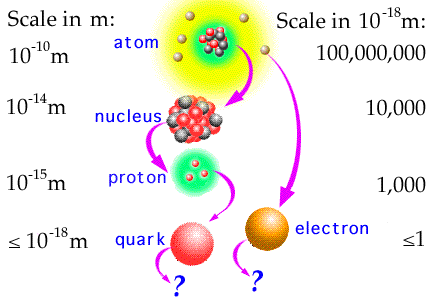
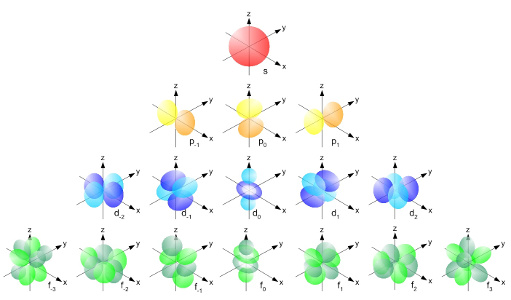
| Atomic particles scale by NASA/GSFC Section 20 | Atomic s, p, d, and f shells by LibreTexts |
Chemistry describes atoms on a proton/electron level and how they interact: Forming molecules and crystals, and conducting electricity. Unfortunately, most chemistry tutorials start from a practical laboratory perspective. In this article, I start with a single proton and develop core ideas of chemical bonds from there.
Disclaimer: I have only the most basic of knowledge about
chemistry whatsoever. If you notice glaring errors, please let me
know under chemistry at the above domain.
Atoms are made up of protons (+neutrons) and electrons. Protons have positive electric charge, electrons negative. Although protons are about 1800x bigger than electrons, their amount of charge is the same. Atoms usually have the same number of protons and electrons (otherwise, they are called ions). Neutrons are just needed to keep the protons from touching each other; superfluous neutrons are possible, and the different variants are called isotopes. Protons and neutrons are packed together in the nucleus, and electrons hover around probabilistically, arranged on so-called shells (each of which has multiple lobes).
 |
 |
|
| Atomic particles scale by NASA/GSFC Section 20 | Atomic s, p, d, and f shells by LibreTexts |
Ok, so we start with one proton (no neutron needed), and add one electron in the innermost shell. Congratulations, you have H (hydrogen)! Now, what can we do with it? Well, each shell has room for a certain number of electrons, and if a shell is not full, it wants more electrons -- and if some other element (=atom type) happens to have those fitting electrons (preferably on fitting shells), we have a merger candidate.
So, which elements do we have? One for each number of protons, listed in the periodic table of elements. H(ydrogen) has 1, He(lium) has 2, Li(thium) has 3, Be(rillium) has 4, B(oron) has 5, C(arbon) has 6, N(itrogen) has 7, O(xygen) has 8, and so on.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Periodic table of elements, simplified subset |
Above, I have green-lighted H (hydrogen, 1 proton), C (carbon, 6 protons), and O (oxygen, 8 protons), because they are fraggin everywhere.
Now, seeing which electrons go into which shells is actually not trivial. Every row in the element table has at least one so-called "s-shell" containing up to 2 electrons, explaining the first row. The second row has (in addition to a second s-shell) another "p-shell" containing up to 6 electrons; same goes for the third row which has 3 s-shells and 2 p-shells in total. Then, the fourth and fifth rows have an additional "d-shell" containing up to 10 electrons, and actually the table has even more rows where a "f-shell" containing up to 14 electrons comes into play.
Trouble is, filling the shells is not quite as easy as it seems. First, the spdf-shells are not always on the same level, and second, they fill up in a diagonal pattern.
In the left image, all looks well until the fourth row, where 3d is stuck between 4s and 4p, which is not the most intuitive pattern. In the right image, we go by row but follow the red arrows first, meaning that the order of filling is 1s, 2s, then 2p, 3s, then 3p, 4s, then 3d, 4p, 5s, and so on. Coming back to H, C, and O, this means that H is 1s1 (meaning on shell 1s there is 1 electron), C with 6 protons is 1s2-2s2-2p2, and O with 8 protons is 1s2-2s2-2p4. And yes, the notation is strange, with the exponent being no real exponent and all this.
As a side note, the rightmost atoms in the periodic table have all their shells full: He (helium, 2 protons) is 1s2, Ne (neon, 10 protons) is 1s2-2s2-2p6 and Ar (argon, 18 protons) is 1s2-2s2-2p6-3s2-3p6. This means they dont feel like reacting to anything, earning them the moniker "noble".
A second side note: The shells really are discrete, which means no electron can go "in between them". Electrons can get excited from their ground state (by shooting with photons or with other electrons), going to a higher (and also discrete) energy level (sometimes energy band), but stay on the same shell. We can think of the excitation as "going faster". When electrons fall back to a lower energy level, they emit a photon with a wavelength of the distance between the levels.
One last thing you need to know: Electrons have quantum spin, which is either "up" or "down", and on each shell electrons have alternating spin. Two electrons with opposite spins can occupy the same orbital, which is why even the (small) s-orbitals can house two electrons. And a spin pair of electrons is more stable than a single one, which means it is harder (takes more heat) to wrestle the first electron from an atom with an even number of electrons.
That about sums it up how we take a bunch of protons (+stuff neutrons between them), and then mash the matching number of electrons onto their shells.
Further reading: What are Chemical Elements good for?.
Enough already, now let's see how we can combine atoms into molecules. Suppose that we take two H (hydrogon, 1s1) atoms and put them into the same closed pressure chamber. To make them bond together as H2 (which is actually the most common way raw H occurs on Earth), a certain energy (pressure, heat, light or electricity) must be applied to allow the "snapping" to a new state, according to the transition state theory. Once that energy is applied, they form a covalent bond, which means both atoms reinforce it.
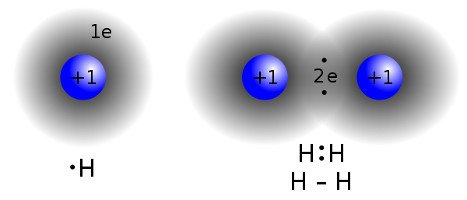 |
| Covalent bond between two hydrogen atoms by Wikipedia |
Now atoms and molecules have potential energy, and they always strive to go to the lowest energy state possible (much like a ball wants do roll downhill). In a lower energy state, bonds are strong and shells are full (in the image above, both H have their first shell full as 1s2). But the point is: when going lower, the difference in energy must go somewhere, and usually this means photon energy is emitted, so the transition reads 2H ⇒ H2+ energy.
It is currently not completely known why some molecule configurations have lower energy states than others, although the abovementioned shell fullness, relative location of shells, and quantum spin most certainly play a role in it. So that is as far as the particle chemistry model goes; from now on, all we can do is develop some intuition.
Let's talk about water, and why it is so damn good at dissolving stuff (e.g. washing your clothes). The H2O (water) molecule consists of one O (oxygen, 1s2-2s2-2p4) which misses 2 electrons on its 2p-shell, and two H (hydrogen, 1s1) which provide 1 electron on their 1s-shell. Usually O exists as O2 and H as H2, which means that considerable heat/pressure needs to be applied to split them into their component H and O. But then, they merge with 2H2 + O2 ⇒ 2H2O + energy, forming a beautifull filled 1s-shell (for H) and 2s/2p-shell (for O).
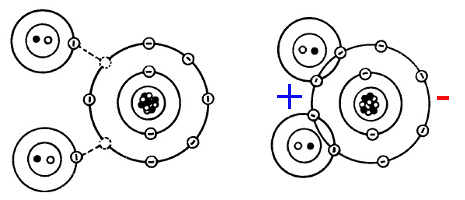 |
| Water molecule bonds by FreeEd |
That is some pretty low energy state, and more photon energy is released than for the 2H⇒H2 transition. Another nice effect is that the previously uniform distribution of charge (electrons can be statistically anywhere on their shell) has now a bias: Since the H electron is a bit farther from O ("because H pulls it" is not the entire truth, but a good intuition) than O's remaining electrons, the latter repel each other stronger than the shared-with-H electrons, pushing the H together; thus the "H side" is slightly electro-positive, and the "O side" is slightly electro-negative.
This means a lot of things: First, water turns in an electricmagnetic field. Second, water can use charge to weakly stick together. Third, whirling water can use charge to attract dirt from your jeans, by drawing either electrons or protons to its positive or negative side.
Electricity encompasses quite a few concepts, but at the most basic level they mean charge potential [C] and moving charge [A], e.g. in a wire.
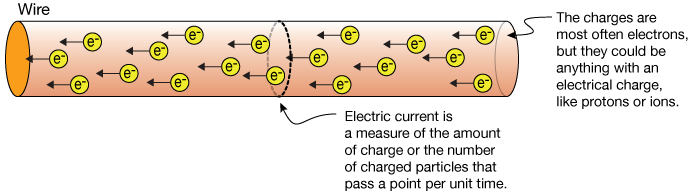 |
| Electrons in a copper wire (protons not shown) by Xaktly |
For charge propagation, metals are easiest, because they share nearly free-floating electrons; water molecules can change directions to propagate charge; regular lattices like carbon very much resist the idea of handing electrons around; and in empty space you need to shoot single electrons or protons around, as in space weather. Depending on the medium, you need to apply more or less Voltage (charge pressure) to get an electron moving.
Intuitively, electricity can be compared to airflow:
Consider the above wire, with perfectly neutral net charge of the copper molecules (same number of protons and electrons). Attach the negative side of a battery (wants to give electrons) to the right end of the wire: One electron pushes against the electrons on the right end with its charge, the push wave propagates at near light speed to the left end of the wire, but there is nowhere to go -- so the wave backfires and the original electron receives an equally hard back-push and has to stay in place. Now attach the positive side of the battery (wants to receive electrons) to the left end of the wire: The wave propagates again, and this time one electron falls out of the left wire end, and the original pusher (electron) can finally enter the wire on the right end.
This is essentially what happens in direct current, as typical for batteries. In toasters et al., alternating current pushes and pulls electrons with a certain frequency (typically 60Hz) back and forth, which combined with the friction in the metal is enough to create heat.
The fusion of two H atoms is stranger than expected. E.g. within the sun, H2 dissolves first into 2H, then into two protons and two electrons. Then the two protons are pressured together under a lot of resistance because the two positive charges repel each other. But if the pressure succeeds in pressing them together, one proton turns into a neutron, releasing among others a positron (anti-electron, positive charge).
| Nuclear fusion of two protons by Wikipedia |
And soon, the anti-electron annihilates with a "normal" electron, and this produces copious amounts of photons.
So in a sense, nuclear fusion (as in fusion power) is outside the scope of this article because it goes lower than basic particles, and into subatomic particles.
I did not want your mind to wander to from "science mode" to "art mode" further above, but now we can enjoy some nice Cartoon art from KCD Studios to close our virtual page...
 |
 |
 |
| Cartoon elements by KCD Studios | ||
Thanks for leaving a part of your attention span here, and have a good day!
EOF (Mar:2021)